Structural Biology of Lipid-Activated Signal Transduction
Thomas Leonard
Group Leader
thomas.leonard@mfpl.ac.at
Phone: +43-1-4277-52205
Campus Vienna Biocenter 5, 1030 Vienna | Room: 1.616

Research
Membranes are sites of intense signaling activity in eukaryotic cells. Essential processes such as autophagy, cytokinesis, exo- and endo- cytosis, and cytoskeletal remodeling depend on signal propagation at cellular membranes. Dysregulation of signal transduction at these sites is the cause of a number of hereditary and non-hereditary diseases, including Coffin-Lowry syndrome, spinocerebellar ataxia, myotonic dystrophy, and various cancers. Over 500 kinases and 130 phosphatases regulate signal transduction by phosphorylating or dephosphorylating their target proteins. Given that the chemistry of phosphoryl transfer is conserved, there is a clear need for compartmentalization of what are essentially the same chemical reactions.
Signal Transduction by Lipid Second Messengers
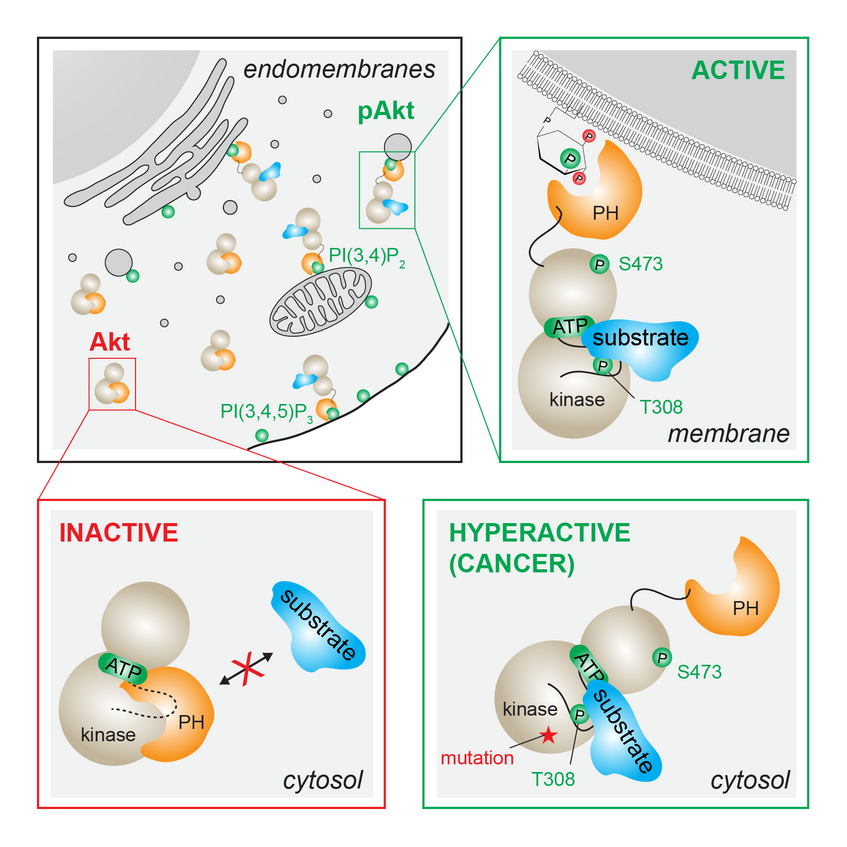
Of the more than 500 kinases, 54 contain known lipid-binding or membrane-interacting domains, and whilst much is known about how these proteins are targeted to cellular membranes, very little is known about how membrane engagement is coupled to signal transduction. We are using a spectrum of biophysical (including X-ray crystallography), biochemical, and cell biological techniques to investigate signal transduction at membranes. We have recently demonstrated the allosteric activation of Akt by the lipid second messengers PI(3,4,5)P3 and PI(3,4)P2 for the first time (Ebner and Lučić et al., Molecular Cell 2017).
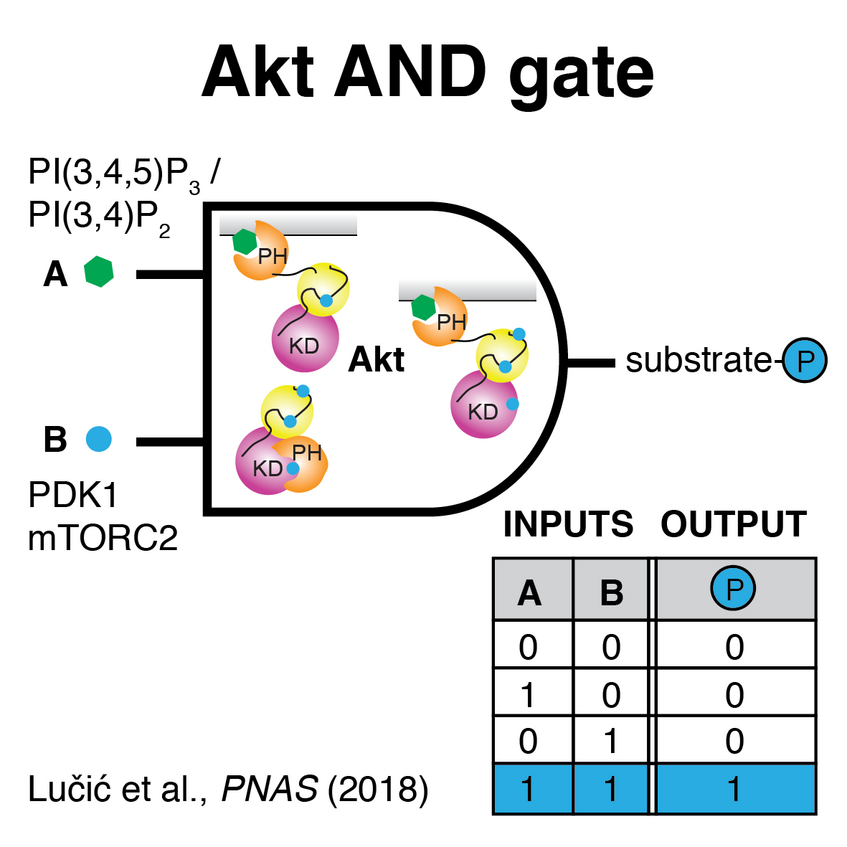
Akt - a Logic Gate Signal Processor
Many of the lipid responsive human protein kinases belong to the AGC family of kinases, of which paradigmatic lipid-activated kinases are Akt and protein kinase C (PKC). In 2017 we demonstrated the allosteric activation of Akt by the lipid second messengers PI(3,4,5)P3 and PI(3,4)P2 for the first time. A mutation in the kinase domain associated with cancer and overgrowth disorders of the brain causes Akt hyperactivation by relieving autoinhibition (Ebner and Lučić et al., Molecular Cell 2017). In our most recent work on Akt, we describe the mechanism of activation in more detail and identify the allosteric coupling between PH domain-mediated autoinhibition and the inactivation of Akt by dephosphorylation (Lučić et al., PNAS 2018; Leonard [Reply to Letter to Editor], PNAS 2018). Conceptually, Akt can be thought of as an AND gate that integrates multiple signals to drive the biological response.
Switching Off with ADP
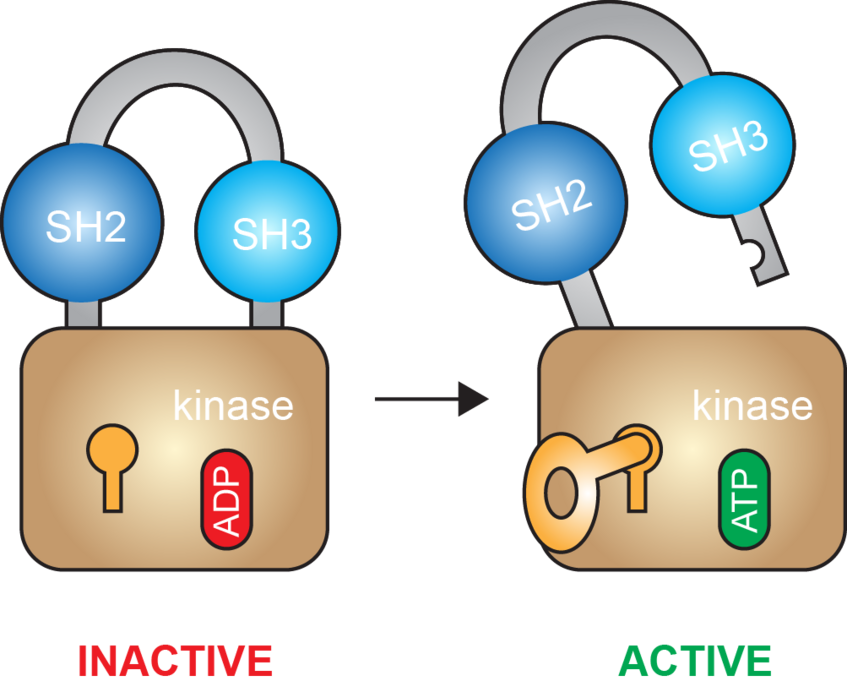
Fundamentally, we aim to describe signal transduction processes with deep mechanistic insight. By combining structure with quantitative in vitro biochemistry on rigorously validated samples, we can probe the structure-function relationship in absolute terms. We have recently applied these principles to study the Tec and Src family tyrosine kinases, work which has revealed a previously unknown switch in nucleotide binding that drives kinase activation (von Raußendorf et al., Sci Rep 2017).
Probing Structure In Vivo
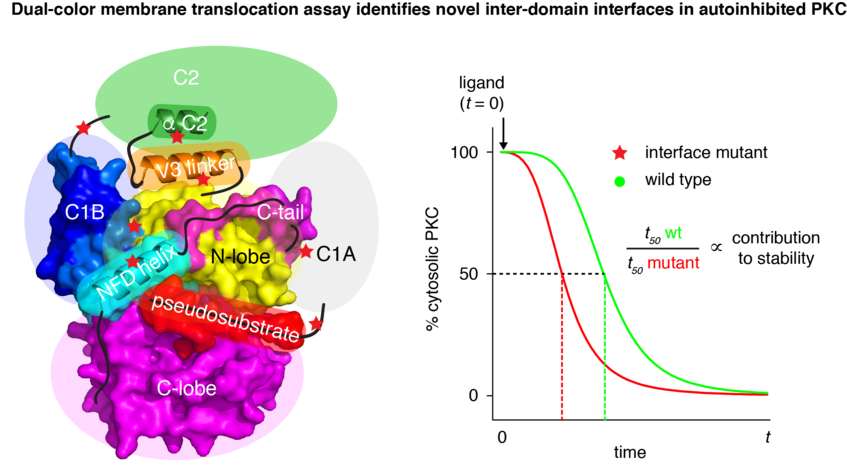
Protein kinase C (PKC) is regulated by multiple membrane binding domains that respond to calcium, phospholipids, and the lipid second messenger diacylglycerol (DAG). PKC is held in an inactive conformation in resting cells by a complex network of intramolecular interactions that sequester its membrane binding domains and prevent substrate binding by its kinase domain. Using a dual color in vivo membrane translocation assay, we have shown that the ten mammalian PKCs adopt a conserved three-dimensional architecture despite domain rearrangements in their primary sequences (Lučić et al., Journal of Molecular Biology, 2016).
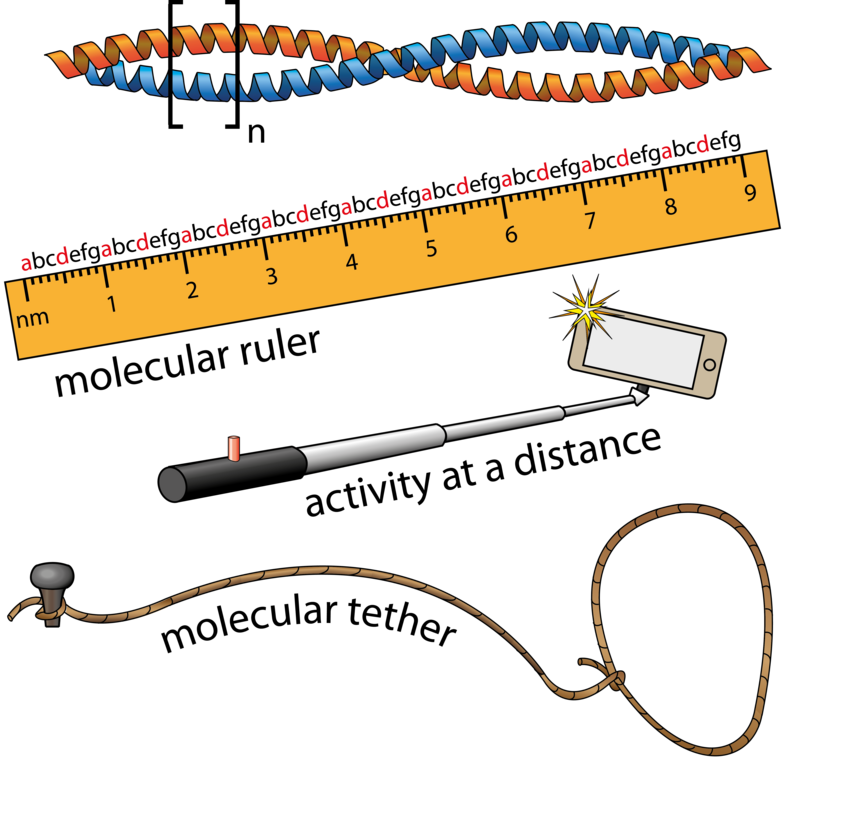
Membrane Scaffolding of Signaling Pathways
We are also interested in how signal transduction pathways are organized. Scaffolding of signaling proteins in the same pathway enhances specificity, promotes signal amplification by reducing noise, and, ultimately, improves signal propagation through the pathway. Membranes act as the scaffolds for many signaling reactions, including those involved in regulation of the actin cytoskeleton (Truebestein et al., Nature Communications 2016). Our studies are aimed at understanding how diverse signals are integrated, how substrate specificity is encoded not just at the kinase level, and the influence of the membrane environment on multi-component signaling hubs. This is an exciting area of research with frontiers in ageing, cancer, metabolic diseases such as diabetes, and obesity.
The Leonard lab invites applications from bright young people fascinated by science. If you are interested in joining a team to work on fundamental questions in biological signal transduction, please get in touch! For further details, please see: https://www.training.vbc.ac.at/phd-programme/
Publications
von Raußendorf, Freia; de Ruiter, Anita; Leonard, Thomas A. (2017). A switch in nucleotide affinity governs activation of the Src and Tec family kinases Scientific reports.;7(1):17405. PMID: 29234112
Michael Ebner, Iva Lučić, Thomas A. Leonard, and Ivan Yudushkin (2017). PI(3,4,5)P3 Engagement Restricts Akt Activity to
Cellular Membranes MOL CELL;65(3):416-431. PMID: 28157504
Truebestein, Linda; Elsner, Daniel J; Fuchs, Elisabeth; Leonard, Thomas A (2015). A molecular ruler regulates cytoskeletal remodelling by the Rho kinases. NAT COMMUN;6:10029. PMID: 26620183
more publications of the group Leonard
Collaborations & Funding

National Scientific Research Fund (FWF) Project
Project title: "Structure, Function, and Regulation of Protein Kinase D" (P 30584)

Hertha Firnberg Postdoctoral Fellowship (FWF)
Dr. Linda Trübestein is a recipient of a Hertha Firnberg Postdoctoral Fellowship (FWF).
Project title: "Bridging the Gap - Regulation of the Cytoskeleton by the DMPK kinases" (T 915)
Doctoral Program "Signaling Mechanisms in Cellular Homeostasis"
The Leonard Group is a member of the special doctoral program "Signaling Mechanisms in Cellular Homeostasis", reviewed and funded by the Austrian Research Fund FWF (start 2017).

National Scientific Research Fund (FWF) Project
Project title: "Lipid-activated kinases in cell shape and motility" (P 28135)

Boehringer Ingelheim Fonds PhD Fellowship
Daniel Elsner is a recipient of the prestigious BIF PhD Fellowship.

DOC Fellowship
Freia von Raussendorf is a recipient of the Austrian Academy of Sciences DOC Fellowship
